The elements were forged in nuclear furnaces inside stars. Ordinary stars like the sun burn hydrogen, two hydrogen atoms colliding together to make a helium atom and releasing energy in the form of heat and light. In dying stars, the hydrogen runs out and the helium atoms fuse together to make the heavier elements. Elements heavier than iron, such as lead and uranium, are only formed when a massive star explodes to become a supernova. The dust left from such an explosion eventually re-forms to make new stars and planets like our Earth. We are all literally made of stardust.
In the ancient world, the elements, or basic constituents of matter, were believed to be earth, air, fire, and water. We now know that there are in fact more than 90 naturally occurring elements, including metals, semi-metals, and non-metals. At ambient temperature, some elements exist as gases, a few as liquids, and many as solids, but their state of matter can change with temperature. As potters we are most interested in the elements in the Earth’s crust that form rocks, as these are also the elements present in clay and glazes. The most abundant are oxygen, silicon, and aluminum, followed by iron, calcium, sodium, potassium, and magnesium (1).
Structure of the Atom
Atoms were once thought to be the smallest units of matter, but we now know they are made up of a number of smaller, subatomic particles. In the center of each atom is the nucleus, which is a cluster of positively charged protons and neutrons, the latter having no electrical charge. Around the central nucleus there is a cloud of negatively charged electrons, arranged in orbitals like planets in the solar system. The inner orbital or shell has 2 electrons, the next can hold 8, the next can hold up to 18 and so on. The electrons in the outermost shell have the highest energy and they can bond with other atoms to form molecules. The electrons are attracted to the positively charged protons in the nucleus and held together by the electromagnetic force. In each atom, there is the same number of electrons as protons so that the overall charge is zero. The number of protons is called the atomic number and is different for each element. The hydrogen atom has only one proton and one electron, while oxygen has 8 protons, 8 neutrons, and 8 electrons, and silicon has 14 protons, 14 neutrons, and 14 electrons (2).
Molecules
The two most common elements in the Earth’s crust are oxygen and silicon. When silicon bonds with oxygen it forms silicon dioxide, or silica, the main constituent of flint, quartz, and sand. It is not surprising that these two elements should also be found in many rocks and minerals, known as silicates. When a third element, aluminum, is added, the resulting mineral is called an alumino-silicate. Clays and feldspars are both types of alumino-silicates, containing silicon, aluminum, and oxygen.
When two atoms bond together, a molecule is formed. This happens either when two similar atoms such as oxygen bond to form O2 gas or when different atoms such as hydrogen and oxygen bond to form water, H2O.
Naming Compounds
Compounds are named using simple conventions. Compounds with two oxygen atoms are named dioxide, for example silicon dioxide. This can also be simplified to silica. An a on the end, as in silica, means combined with oxygen. An ate on the end, as in carbonate, means combined with both carbon and oxygen or in silicate, combined with both silicon and oxygen. Some molecules are known by several names, for example, lime (as in limestone) is another name for calcium carbonate. The names sometimes get more complicated, for example, aluminum sesquioxide means two atoms of aluminum to every three atoms of oxygen. This is written as Al2O3 and is also known simply as aluminum oxide or alumina.
Electrons and Orbitals
Only the electrons in the outer shell or orbital of an atom can take part in bonding. Silicon has four electrons available (there are another ten in two inner shells). These outer electrons are called valence electrons. When two atoms share each other’s electrons, they form a bond, called a covalent bond. Every silicon atom needs four oxygen atoms to form a molecule. Rather than forming single molecules, the silica molecules join together to form a giant network structure in which each silicon atom is joined to four oxygen atoms in a tetrahedron. Because each oxygen atom is shared by two silicon atoms, the overall formula is SiO2.
In other molecules, such as sodium oxide, there is a different type of bonding called ionic bonding, where an atom donates one of its electrons to another. The sodium atom gives up the single electron in its outer shell to make a sodium ion with a single positive charge. The electron fills a gap in the outer shell of the oxygen atom, which has six outer electrons. To complete the shell, the oxygen atom needs eight electrons, so it bonds with two sodium ions, each of which gives an electron to the oxygen’s outer shell (3). However, most chemical bonds are partly ionic and partly covalent. The sodium oxide molecule has the formula Na2O, where two sodium atoms are bonded to every oxygen atom. When this reacts with water, it becomes a strong alkali, sodium hydroxide, NaOH. For this reason, sodium and the similar metals lithium and potassium are called alkali metals. When sodium oxide reacts with silica, it breaks up the silica network structure, acting as a flux to melt the silica.
The Potter’s Periodic Table
The periodic table explains the chemical and physical properties of the different materials used by potters (4). It is the basis for understanding the whole of chemistry. However, we are primarily interested in the elements useful to potters, which include silicon, aluminum, the alkali metals and alkaline earth metals, as well as the transition metals.
During the nineteenth century, scientists began to notice that groups of elements had similar properties. Not all the elements had yet been discovered, but when they were lined up in order of increasing atomic number in seven rows, similarities between groups became clear. We know now that is because the elements in a group all have the same number of electrons in their outer shell, so they all react in similar ways.
In the periodic table, the elements are listed in order of ascending atomic number. There are seven rows of elements and eighteen columns. The elements in each column, called a group, have similar properties. Important groups for the potter are group 1, the alkali metals (sodium, potassium etc.), and group 2, the alkaline earth metals (calcium, magnesium etc.). These are used as fluxes in glazes. All the elements on the left-hand side and middle of the periodic table are metals. The ones at the bottom are known as heavy metals as they have more protons and neutrons and therefore greater atomic weights. Many of the metals discovered since the early nineteenth century have names ending in ‘-ium’, for example, sodium, calcium, barium. Their oxides form alkaline solutions when dissolved in water. The oxides, rather than the metals, are of most interest to the potter as they are the constituents of clay and glazes.
The central block of the periodic table contains the transition metals. These can have several oxidation states and are important in producing colored glazes, particularly the metals along the top row: chromium, manganese, iron, cobalt, nickel, and copper. The two rows inserted at the bottom are known as the lanthanides and the actinides, and some can also produce colors in glass and glazes. Uranium is the heaviest naturally occurring element and can produce yellow in glazes, although it is no longer used owing to its radioactivity. The elements beyond uranium can only be synthesized in nuclear reactors and decay radioactively into other elements. Lead is the heaviest stable, non-radioactive element.
On the right-hand side of the periodic table is a diagonal line, which divides metals from non-metals. The elements on this line include boron, silicon, and arsenic. They are known as metalloids and are semiconductors, which means they will only conduct electricity under certain conditions. Silicon is the basis of modern electronics and computers. Some of the elements most useful to potters also lie on or near this dividing line and include silicon and boron, which are both glass formers although the latter is mainly used as a flux in low-temperature glazes and in heat-resistant borosilicate glass. Aluminum is next to silicon, found in clay and used as a stabilizer in glazes, preventing them from becoming too runny when fired. On the right-hand side of silicon is phosphorus, used as a flux in glazes and in bone china. Antimony and selenium are used in small amounts as yellow and red colorants in glazes.
The top right-hand corner of the periodic table contains oxygen, the halogens fluorine and chlorine, and the noble gases helium and neon. Oxygen is present in all clay and glazes, usually combined with silicon and aluminum as oxides. Fluorine is of interest to the potter, as it is often given off in the kiln from decomposing fluorspar, a mineral found in Cornwall Stone.
The far left of the periodic table is highly alkaline and the right is highly acidic. The strength of the alkali decreases from left to right, while the strength of the acid increases. For example, along the third row, sodium oxide (Na2O) is highly alkaline, magnesium oxide (MgO) is alkaline, aluminum oxide (Al2O3) is amphoteric, silicon dioxide (SiO2) is acidic, and phosphorus pentoxide (P2O5) is highly acidic. In ceramic glazes and vitreous clay bodies, the alkaline and acidic oxides react together and melt. The alkaline metal oxides are fluxes, which react with the acidic glass former, silica. The elements in the central block are amphoteric (able to react as an acid or base/alkaline), although many show slightly alkaline or acidic properties. Their oxides are used in glazes as supplementary fluxes, stabilizers, opacifiers, and colorants.
Metals
Group 1, The Alkali Metals: includes lithium (Li), sodium (Na) and potassium (K) (5). They are extremely reactive metals and react vigorously with water. They are not found naturally in their metallic state but only in combination with other elements. Their oxides are strong fluxes and help to melt the silica in a glaze. Their fluxing ability decreases down the group. The alkali metal oxides are found in feldspars and they are also available in the more soluble carbonate form. The oxides have the chemical formula R2O, where R is the metal and O is oxygen.
Lithium is the least reactive of the alkali metals but its oxide has the highest fluxing power in glazes. Lithium oxide melts at a higher temperature than sodium and potassium but once melted, forms a more fluid melt. Lithium has a small atom size and is the lightest metal, so compared to sodium, weight for weight, only a small amount is needed to flux a glaze. Lithium oxide has a lower expansion rate than the other alkali metals and is used in shock-resistant, heatproof ovenware.
Sodium oxide is a stronger flux than potassium oxide. It has a higher melting point than potassium oxide but produces a more fluid melt. However, it is volatile above 2192°F (1200°C) and can cause the glaze to bubble. It has a high expansion rate and can cause crazing in glazes.
Potassium oxide begins to melt at a slightly lower temperature but has a wider firing range than sodium oxide, so can be used at higher firing temperatures. It produces a more viscous glaze, which is harder and more resistant to scratching than a sodium-based glaze.
Group 2, The Alkaline Earth Metals (6): includes magnesium (Mg), calcium (Ca), strontium (Sr) and barium (Ba). The oxides of the alkaline earth metals are used as secondary fluxes in glazes. They have surprisingly high melting temperatures, but act in eutectic mixtures to lower the melting point of the glaze. They also stabilize the glaze and make it more durable. The oxides have the chemical formula RO, where R is the metal and O is oxygen.
Calcium oxide is the most widely available alkaline earth oxide. It is used to make matte glazes and as a flux at firing temperatures above 2012°F (1100°C).
Magnesium oxide is used to make satin matte glazes and has a low expansion rate, useful for correcting crazing. It has a higher melting point than calcium oxide and is an active flux above 2138°F (1170°C).
Barium oxide can be used as a flux above 2147°F (1175°C) and to make matte glazes. However, its source material, barium carbonate is toxic. Strontium oxide can be used as an alternative, non-toxic flux above 1994°F (1090°C). It has properties similar to calcium and sits between calcium and barium in the group. Barium and strontium mattes give brighter colors than magnesium and calcium, which tend to bleach out colors. Barium and strontium can be used at low-fire temperatures if they have first been made into a frit.
Transition Metals
The transition metals can each have several different oxidation states, meaning they can combine with different numbers of oxygen atoms, depending on the surrounding conditions. They have an incomplete inner shell of electrons, which allows the electrons to move around between energy levels. The transition metals most important to potters are called the coloring oxides. These are found on the top row of the transition metal block and are the smallest-sized transition metal atoms. They include titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), and copper (Cu) (7). Chromium oxide has the formula Cr2O3, and is amphoteric with acidic tendencies, while cobalt, nickel and copper oxides have the formula RO and are amphoteric with alkaline tendencies. Manganese and iron oxide have several valences, for example red (ferric) iron oxide Fe2O3, is acidic and refractory, while black (ferrous) iron oxide FeO is alkaline and acts as a flux. Copper oxide can give dramatically different colors in different conditions, including green in oxidation and ox-blood red in reduction (8).
Although it is in the same block, zinc (Zn) oxide is not technically a transition metal as its inner shell is completely filled with electrons. It is used not as a colorant, but as a flux in mid-temperature glazes. In the next row of the periodic table, of interest to potters are zirconium (Zr), used to opacify glazes, and cadmium (Cd), used in red and yellow glaze stains. Molybdenum (Mo) and Tungsten (W) are used in iridescent crystalline glazes. Silver (Ag), gold (Au), and platinum (Pt) can be used by potters in metallic lusters.
Rare Earths
The rare earth elements cerium (Ce), praseodymium (Pr), neodymium (Nd), and erbium (Er) (9) are used to color glass and, in the case of praseodymium, to make a yellow stain for glazes. Neodymium (which means new twin) was found mixed with praseodymium (meaning green twin, from the Greek prasios didymos) and the pure oxides give respectively a violet and green color in glazes. The rare earths are also known as the lanthanides as they follow the element lanthanum in the periodic table. They are refractory and have the theoretical formulae CeO2, PrO2, Nd2O3, and Er2O3. They are weak colorants but can be used in glazes on porcelain. The insoluble oxides are not toxic, although the firing fumes should be avoided. Some of the other lanthanides can give bright fluorescent glaze colors, but only under ultraviolet light. Though not particularly rare, they are currently only mined in China and have recently become very expensive. They are extracted from the minerals monazite, which is radioactive, and bastnäsite.
The bottom row of the periodic table, the actinide series, contains uranium (U), which was used in the mid-20th century to make bright yellow and orange glazes, but is now unobtainable owing to its radioactivity.
Poor metals
The post-transition metals, also known as poor metals (10), look silvery like metals, but are weak metals, with low mechanical strength. They are chemically close to the non-metals and, being near the bottom of the periodic table, are relatively soft, with low melting points.
Aluminum is an abundant metal in the Earth's crust. Aluminum oxide, or alumina, is found together with silica in clay, which is an alumino-silicate. Alumina is used to make glazes more viscous in the melt to prevent them running off the pot. Aluminum oxide has the formula Al2O3 and is amphoteric, neither alkaline nor acidic. It has an extremely high melting point of 3722°F (2050°C). In its crystalline form, alumina forms a very hard mineral called corundum or sapphire, used to make scratch-resistant watch faces. It is the second hardest mineral next to diamond.
Tin (Sn) is a metal also found in this group of elements. Tin oxide (SnO2) is an acidic oxide used as an opacifier in glazes. It was originally used to cover coarse clay bodies with a white tin glaze, imitating the look of Chinese porcelain.
Lead oxide (PbO) was once used as the main flux in low-temperature glazes but its use has now diminished owing to its toxicity and tendency to accumulate in the body. Lead bisilicate and sesquisilicate frits are still used in the UK and around the world, but have been superseded by borosilicate frits in the US.
Bismuth (Bi) is another metal found in this group, used by potters in mother-of-pearl lusters.
Semi-Metals
This diagonal group is known as the metalloids. They are generally hard and brittle and are semiconductors.
Boron (B) falls on the boundary between metals and non-metals. Its oxide can be used as a flux and, used together with sodium and calcium, it has replaced lead in earthenware glazes. Boric oxide is also a glass former and has a very low expansion rate. It is used to make heatproof borosilicate lab glass. Like all non-metals, it forms an acidic oxide, which has the chemical formula B2O3.
Silicon (Si) is semiconductor and is a brittle, silvery-gray semi-metal used to make silicon chips in computers. Silicon dioxide (SiO2), or silica, forms over 60% of the Earth’s crust and is the basis of silicate minerals; rocks, clays, and glazes. The name silicon comes from the Latin silex meaning hard stone or flint. It was originally named silicium, suggesting a metal, but the name was changed to silicon, which sounds more like the non-metals carbon and boron.
Silica melts to form a glass but has a high melting point of 3110°F (1710°C). However, if alumina is added in certain proportions, the resulting mixture will have a lower melting point (known as a eutectic) than either of the pure constituents. Additional fluxes are required to melt it at the temperatures reached in a kiln. Silica is an acidic oxide, which reacts with alkali metal fluxes.
Antimony (Sb), a semi-metal, can be used in glazes, usually together with lead as a colorant found in low-temperature yellow glazes. This was the bright yellow traditionally used on tin-glazed earthenware or majolica.Germanium, arsenic, and tellurium are present in trace amounts with other glaze colorants. They are used mainly in the semiconductor industry.
Non-Metals
The non-metals form acidic oxides. The acids become progressively stronger from left to right along the period, for example, phosphoric acid, sulfuric acid, and hydrochloric acid (12). Hydrofluoric acid is the strongest acid. When fluorine gas is given off from some clays during firing, it dissolves in water condensed on the windows and can etch the window glass in the studio.
Carbon (C) is present as lignite (an intermediate between peat and coal) in fireclays and ball clays, and in carbonates used in glazes but it turns into carbon dioxide gas on heating and escapes from the kiln. Carbon dioxide dissolves in water to make a weak acid.
Phosphorus (P) is a non-metal and forms an acidic oxide. It is a glass former and is used with calcium in small quantities as a flux and opacifier in stoneware glazes, and in larger quantities in bone china. It is found in wood ash and bone ash (calcium phosphate).
Oxygen (O), Sulfur (S), Selenium (Se): Oxygen is very reactive and is found in most glaze materials, either in the form of an oxide or carbonate, or combined with silicon in a silicate. All the chemical interactions in clay and glazes during firing involve oxides.
Sulfur (or sulphur in the UK) (S) is sometimes present in clays and is given off during firing as sulfur dioxide. It is also present in some yellow glaze stains containing cadmium sulfide.
Selenium (Se) can also be used in glazes, usually together with cadmium as a colorant found in orange and red stains.
the authorLinda Bloomfield studied engineering at Warwick University, with a year at MIT during her PhD studies. She spent a year in Tsukuba, Japan, working for NEC (Nippon Electric Company) and returned to London to work as a researcher at Imperial College. She started her pottery career while living in California, and became familiar with US potters’ materials. She now lives in London, where she makes porcelain tableware and writes pottery books.
We understand your email address is private. You will receive emails and newsletters from Ceramic Arts Network. We will never share your information except as outlined in our privacy policy. You can unsubscribe at any time.
Please enjoy this complimentary article for the month.
For unlimited access to Ceramics Monthly premium content, please subscribe.
We understand your email address is private. You will receive emails and newsletters from Ceramic Arts Network. We will never share your information except as outlined in our privacy policy. You can unsubscribe at any time.
Subscribe to Ceramics Monthly
The Elements
The elements were forged in nuclear furnaces inside stars. Ordinary stars like the sun burn hydrogen, two hydrogen atoms colliding together to make a helium atom and releasing energy in the form of heat and light. In dying stars, the hydrogen runs out and the helium atoms fuse together to make the heavier elements. Elements heavier than iron, such as lead and uranium, are only formed when a massive star explodes to become a supernova. The dust left from such an explosion eventually re-forms to make new stars and planets like our Earth. We are all literally made of stardust.
In the ancient world, the elements, or basic constituents of matter, were believed to be earth, air, fire, and water. We now know that there are in fact more than 90 naturally occurring elements, including metals, semi-metals, and non-metals. At ambient temperature, some elements exist as gases, a few as liquids, and many as solids, but their state of matter can change with temperature. As potters we are most interested in the elements in the Earth’s crust that form rocks, as these are also the elements present in clay and glazes. The most abundant are oxygen, silicon, and aluminum, followed by iron, calcium, sodium, potassium, and magnesium (1).
Structure of the Atom
Atoms were once thought to be the smallest units of matter, but we now know they are made up of a number of smaller, subatomic particles. In the center of each atom is the nucleus, which is a cluster of positively charged protons and neutrons, the latter having no electrical charge. Around the central nucleus there is a cloud of negatively charged electrons, arranged in orbitals like planets in the solar system. The inner orbital or shell has 2 electrons, the next can hold 8, the next can hold up to 18 and so on. The electrons in the outermost shell have the highest energy and they can bond with other atoms to form molecules. The electrons are attracted to the positively charged protons in the nucleus and held together by the electromagnetic force. In each atom, there is the same number of electrons as protons so that the overall charge is zero. The number of protons is called the atomic number and is different for each element. The hydrogen atom has only one proton and one electron, while oxygen has 8 protons, 8 neutrons, and 8 electrons, and silicon has 14 protons, 14 neutrons, and 14 electrons (2).
Molecules
The two most common elements in the Earth’s crust are oxygen and silicon. When silicon bonds with oxygen it forms silicon dioxide, or silica, the main constituent of flint, quartz, and sand. It is not surprising that these two elements should also be found in many rocks and minerals, known as silicates. When a third element, aluminum, is added, the resulting mineral is called an alumino-silicate. Clays and feldspars are both types of alumino-silicates, containing silicon, aluminum, and oxygen.
When two atoms bond together, a molecule is formed. This happens either when two similar atoms such as oxygen bond to form O2 gas or when different atoms such as hydrogen and oxygen bond to form water, H2O.
Naming Compounds
Compounds are named using simple conventions. Compounds with two oxygen atoms are named dioxide, for example silicon dioxide. This can also be simplified to silica. An a on the end, as in silica, means combined with oxygen. An ate on the end, as in carbonate, means combined with both carbon and oxygen or in silicate, combined with both silicon and oxygen. Some molecules are known by several names, for example, lime (as in limestone) is another name for calcium carbonate. The names sometimes get more complicated, for example, aluminum sesquioxide means two atoms of aluminum to every three atoms of oxygen. This is written as Al2O3 and is also known simply as aluminum oxide or alumina.
Electrons and Orbitals
Only the electrons in the outer shell or orbital of an atom can take part in bonding. Silicon has four electrons available (there are another ten in two inner shells). These outer electrons are called valence electrons. When two atoms share each other’s electrons, they form a bond, called a covalent bond. Every silicon atom needs four oxygen atoms to form a molecule. Rather than forming single molecules, the silica molecules join together to form a giant network structure in which each silicon atom is joined to four oxygen atoms in a tetrahedron. Because each oxygen atom is shared by two silicon atoms, the overall formula is SiO2.
In other molecules, such as sodium oxide, there is a different type of bonding called ionic bonding, where an atom donates one of its electrons to another. The sodium atom gives up the single electron in its outer shell to make a sodium ion with a single positive charge. The electron fills a gap in the outer shell of the oxygen atom, which has six outer electrons. To complete the shell, the oxygen atom needs eight electrons, so it bonds with two sodium ions, each of which gives an electron to the oxygen’s outer shell (3). However, most chemical bonds are partly ionic and partly covalent. The sodium oxide molecule has the formula Na2O, where two sodium atoms are bonded to every oxygen atom. When this reacts with water, it becomes a strong alkali, sodium hydroxide, NaOH. For this reason, sodium and the similar metals lithium and potassium are called alkali metals. When sodium oxide reacts with silica, it breaks up the silica network structure, acting as a flux to melt the silica.
The Potter’s Periodic Table
The periodic table explains the chemical and physical properties of the different materials used by potters (4). It is the basis for understanding the whole of chemistry. However, we are primarily interested in the elements useful to potters, which include silicon, aluminum, the alkali metals and alkaline earth metals, as well as the transition metals.
During the nineteenth century, scientists began to notice that groups of elements had similar properties. Not all the elements had yet been discovered, but when they were lined up in order of increasing atomic number in seven rows, similarities between groups became clear. We know now that is because the elements in a group all have the same number of electrons in their outer shell, so they all react in similar ways.
In the periodic table, the elements are listed in order of ascending atomic number. There are seven rows of elements and eighteen columns. The elements in each column, called a group, have similar properties. Important groups for the potter are group 1, the alkali metals (sodium, potassium etc.), and group 2, the alkaline earth metals (calcium, magnesium etc.). These are used as fluxes in glazes. All the elements on the left-hand side and middle of the periodic table are metals. The ones at the bottom are known as heavy metals as they have more protons and neutrons and therefore greater atomic weights. Many of the metals discovered since the early nineteenth century have names ending in ‘-ium’, for example, sodium, calcium, barium. Their oxides form alkaline solutions when dissolved in water. The oxides, rather than the metals, are of most interest to the potter as they are the constituents of clay and glazes.
The central block of the periodic table contains the transition metals. These can have several oxidation states and are important in producing colored glazes, particularly the metals along the top row: chromium, manganese, iron, cobalt, nickel, and copper. The two rows inserted at the bottom are known as the lanthanides and the actinides, and some can also produce colors in glass and glazes. Uranium is the heaviest naturally occurring element and can produce yellow in glazes, although it is no longer used owing to its radioactivity. The elements beyond uranium can only be synthesized in nuclear reactors and decay radioactively into other elements. Lead is the heaviest stable, non-radioactive element.
On the right-hand side of the periodic table is a diagonal line, which divides metals from non-metals. The elements on this line include boron, silicon, and arsenic. They are known as metalloids and are semiconductors, which means they will only conduct electricity under certain conditions. Silicon is the basis of modern electronics and computers. Some of the elements most useful to potters also lie on or near this dividing line and include silicon and boron, which are both glass formers although the latter is mainly used as a flux in low-temperature glazes and in heat-resistant borosilicate glass. Aluminum is next to silicon, found in clay and used as a stabilizer in glazes, preventing them from becoming too runny when fired. On the right-hand side of silicon is phosphorus, used as a flux in glazes and in bone china. Antimony and selenium are used in small amounts as yellow and red colorants in glazes.
The top right-hand corner of the periodic table contains oxygen, the halogens fluorine and chlorine, and the noble gases helium and neon. Oxygen is present in all clay and glazes, usually combined with silicon and aluminum as oxides. Fluorine is of interest to the potter, as it is often given off in the kiln from decomposing fluorspar, a mineral found in Cornwall Stone.
The far left of the periodic table is highly alkaline and the right is highly acidic. The strength of the alkali decreases from left to right, while the strength of the acid increases. For example, along the third row, sodium oxide (Na2O) is highly alkaline, magnesium oxide (MgO) is alkaline, aluminum oxide (Al2O3) is amphoteric, silicon dioxide (SiO2) is acidic, and phosphorus pentoxide (P2O5) is highly acidic. In ceramic glazes and vitreous clay bodies, the alkaline and acidic oxides react together and melt. The alkaline metal oxides are fluxes, which react with the acidic glass former, silica. The elements in the central block are amphoteric (able to react as an acid or base/alkaline), although many show slightly alkaline or acidic properties. Their oxides are used in glazes as supplementary fluxes, stabilizers, opacifiers, and colorants.
Metals
Group 1, The Alkali Metals: includes lithium (Li), sodium (Na) and potassium (K) (5). They are extremely reactive metals and react vigorously with water. They are not found naturally in their metallic state but only in combination with other elements. Their oxides are strong fluxes and help to melt the silica in a glaze. Their fluxing ability decreases down the group. The alkali metal oxides are found in feldspars and they are also available in the more soluble carbonate form. The oxides have the chemical formula R2O, where R is the metal and O is oxygen.
Lithium is the least reactive of the alkali metals but its oxide has the highest fluxing power in glazes. Lithium oxide melts at a higher temperature than sodium and potassium but once melted, forms a more fluid melt. Lithium has a small atom size and is the lightest metal, so compared to sodium, weight for weight, only a small amount is needed to flux a glaze. Lithium oxide has a lower expansion rate than the other alkali metals and is used in shock-resistant, heatproof ovenware.
Sodium oxide is a stronger flux than potassium oxide. It has a higher melting point than potassium oxide but produces a more fluid melt. However, it is volatile above 2192°F (1200°C) and can cause the glaze to bubble. It has a high expansion rate and can cause crazing in glazes.
Potassium oxide begins to melt at a slightly lower temperature but has a wider firing range than sodium oxide, so can be used at higher firing temperatures. It produces a more viscous glaze, which is harder and more resistant to scratching than a sodium-based glaze.
Group 2, The Alkaline Earth Metals (6): includes magnesium (Mg), calcium (Ca), strontium (Sr) and barium (Ba). The oxides of the alkaline earth metals are used as secondary fluxes in glazes. They have surprisingly high melting temperatures, but act in eutectic mixtures to lower the melting point of the glaze. They also stabilize the glaze and make it more durable. The oxides have the chemical formula RO, where R is the metal and O is oxygen.
Calcium oxide is the most widely available alkaline earth oxide. It is used to make matte glazes and as a flux at firing temperatures above 2012°F (1100°C).
Magnesium oxide is used to make satin matte glazes and has a low expansion rate, useful for correcting crazing. It has a higher melting point than calcium oxide and is an active flux above 2138°F (1170°C).
Barium oxide can be used as a flux above 2147°F (1175°C) and to make matte glazes. However, its source material, barium carbonate is toxic. Strontium oxide can be used as an alternative, non-toxic flux above 1994°F (1090°C). It has properties similar to calcium and sits between calcium and barium in the group. Barium and strontium mattes give brighter colors than magnesium and calcium, which tend to bleach out colors. Barium and strontium can be used at low-fire temperatures if they have first been made into a frit.
Transition Metals
The transition metals can each have several different oxidation states, meaning they can combine with different numbers of oxygen atoms, depending on the surrounding conditions. They have an incomplete inner shell of electrons, which allows the electrons to move around between energy levels. The transition metals most important to potters are called the coloring oxides. These are found on the top row of the transition metal block and are the smallest-sized transition metal atoms. They include titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), and copper (Cu) (7). Chromium oxide has the formula Cr2O3, and is amphoteric with acidic tendencies, while cobalt, nickel and copper oxides have the formula RO and are amphoteric with alkaline tendencies. Manganese and iron oxide have several valences, for example red (ferric) iron oxide Fe2O3, is acidic and refractory, while black (ferrous) iron oxide FeO is alkaline and acts as a flux. Copper oxide can give dramatically different colors in different conditions, including green in oxidation and ox-blood red in reduction (8).
Although it is in the same block, zinc (Zn) oxide is not technically a transition metal as its inner shell is completely filled with electrons. It is used not as a colorant, but as a flux in mid-temperature glazes. In the next row of the periodic table, of interest to potters are zirconium (Zr), used to opacify glazes, and cadmium (Cd), used in red and yellow glaze stains. Molybdenum (Mo) and Tungsten (W) are used in iridescent crystalline glazes. Silver (Ag), gold (Au), and platinum (Pt) can be used by potters in metallic lusters.
Rare Earths
The rare earth elements cerium (Ce), praseodymium (Pr), neodymium (Nd), and erbium (Er) (9) are used to color glass and, in the case of praseodymium, to make a yellow stain for glazes. Neodymium (which means new twin) was found mixed with praseodymium (meaning green twin, from the Greek prasios didymos) and the pure oxides give respectively a violet and green color in glazes. The rare earths are also known as the lanthanides as they follow the element lanthanum in the periodic table. They are refractory and have the theoretical formulae CeO2, PrO2, Nd2O3, and Er2O3. They are weak colorants but can be used in glazes on porcelain. The insoluble oxides are not toxic, although the firing fumes should be avoided. Some of the other lanthanides can give bright fluorescent glaze colors, but only under ultraviolet light. Though not particularly rare, they are currently only mined in China and have recently become very expensive. They are extracted from the minerals monazite, which is radioactive, and bastnäsite.
The bottom row of the periodic table, the actinide series, contains uranium (U), which was used in the mid-20th century to make bright yellow and orange glazes, but is now unobtainable owing to its radioactivity.
Poor metals
The post-transition metals, also known as poor metals (10), look silvery like metals, but are weak metals, with low mechanical strength. They are chemically close to the non-metals and, being near the bottom of the periodic table, are relatively soft, with low melting points.
Aluminum is an abundant metal in the Earth's crust. Aluminum oxide, or alumina, is found together with silica in clay, which is an alumino-silicate. Alumina is used to make glazes more viscous in the melt to prevent them running off the pot. Aluminum oxide has the formula Al2O3 and is amphoteric, neither alkaline nor acidic. It has an extremely high melting point of 3722°F (2050°C). In its crystalline form, alumina forms a very hard mineral called corundum or sapphire, used to make scratch-resistant watch faces. It is the second hardest mineral next to diamond.
Tin (Sn) is a metal also found in this group of elements. Tin oxide (SnO2) is an acidic oxide used as an opacifier in glazes. It was originally used to cover coarse clay bodies with a white tin glaze, imitating the look of Chinese porcelain.
Lead oxide (PbO) was once used as the main flux in low-temperature glazes but its use has now diminished owing to its toxicity and tendency to accumulate in the body. Lead bisilicate and sesquisilicate frits are still used in the UK and around the world, but have been superseded by borosilicate frits in the US.
Bismuth (Bi) is another metal found in this group, used by potters in mother-of-pearl lusters.
Semi-Metals
This diagonal group is known as the metalloids. They are generally hard and brittle and are semiconductors.
Boron (B) falls on the boundary between metals and non-metals. Its oxide can be used as a flux and, used together with sodium and calcium, it has replaced lead in earthenware glazes. Boric oxide is also a glass former and has a very low expansion rate. It is used to make heatproof borosilicate lab glass. Like all non-metals, it forms an acidic oxide, which has the chemical formula B2O3.
Silicon (Si) is semiconductor and is a brittle, silvery-gray semi-metal used to make silicon chips in computers. Silicon dioxide (SiO2), or silica, forms over 60% of the Earth’s crust and is the basis of silicate minerals; rocks, clays, and glazes. The name silicon comes from the Latin silex meaning hard stone or flint. It was originally named silicium, suggesting a metal, but the name was changed to silicon, which sounds more like the non-metals carbon and boron.
Silica melts to form a glass but has a high melting point of 3110°F (1710°C). However, if alumina is added in certain proportions, the resulting mixture will have a lower melting point (known as a eutectic) than either of the pure constituents. Additional fluxes are required to melt it at the temperatures reached in a kiln. Silica is an acidic oxide, which reacts with alkali metal fluxes.
Antimony (Sb), a semi-metal, can be used in glazes, usually together with lead as a colorant found in low-temperature yellow glazes. This was the bright yellow traditionally used on tin-glazed earthenware or majolica.Germanium, arsenic, and tellurium are present in trace amounts with other glaze colorants. They are used mainly in the semiconductor industry.
Non-Metals
The non-metals form acidic oxides. The acids become progressively stronger from left to right along the period, for example, phosphoric acid, sulfuric acid, and hydrochloric acid (12). Hydrofluoric acid is the strongest acid. When fluorine gas is given off from some clays during firing, it dissolves in water condensed on the windows and can etch the window glass in the studio.
Carbon (C) is present as lignite (an intermediate between peat and coal) in fireclays and ball clays, and in carbonates used in glazes but it turns into carbon dioxide gas on heating and escapes from the kiln. Carbon dioxide dissolves in water to make a weak acid.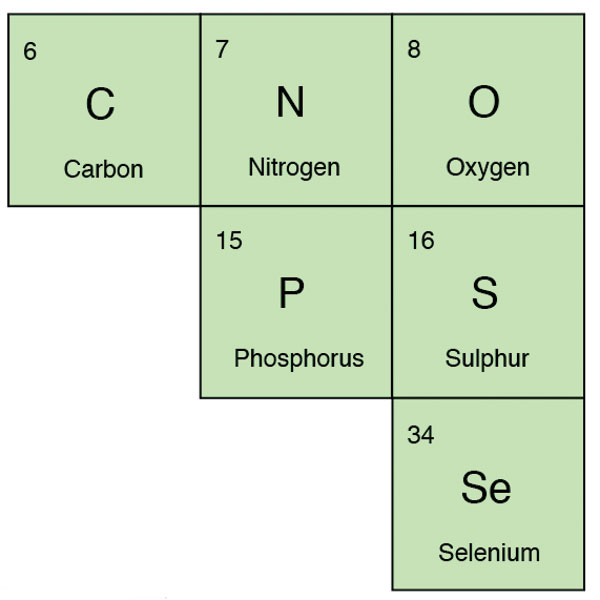
Phosphorus (P) is a non-metal and forms an acidic oxide. It is a glass former and is used with calcium in small quantities as a flux and opacifier in stoneware glazes, and in larger quantities in bone china. It is found in wood ash and bone ash (calcium phosphate).
Oxygen (O), Sulfur (S), Selenium (Se): Oxygen is very reactive and is found in most glaze materials, either in the form of an oxide or carbonate, or combined with silicon in a silicate. All the chemical interactions in clay and glazes during firing involve oxides.
Sulfur (or sulphur in the UK) (S) is sometimes present in clays and is given off during firing as sulfur dioxide. It is also present in some yellow glaze stains containing cadmium sulfide.
Selenium (Se) can also be used in glazes, usually together with cadmium as a colorant found in orange and red stains.
the authorLinda Bloomfield studied engineering at Warwick University, with a year at MIT during her PhD studies. She spent a year in Tsukuba, Japan, working for NEC (Nippon Electric Company) and returned to London to work as a researcher at Imperial College. She started her pottery career while living in California, and became familiar with US potters’ materials. She now lives in London, where she makes porcelain tableware and writes pottery books.
Unfamiliar with any terms in this article? Browse our glossary of pottery terms!
Click the cover image to return to the Table of Contents